|

The
World Surf League have joined forces with Columbia University in the fight
against ocean acidification. The focus is on coral reefs that are being
bleached. The level of bleaching is an indicator of just how bad the
oceans are as a result of climate
change.
Oh
yes, once again we
are in a lot of trouble. Why? Because we don't plan ahead, or rather
governments act retrospectively in response to negative human
exploitation of the planet. That would be okay, but for the huge delay is
reacting to what amounts to massive oceanic slaughter. The pattern is the
same for plastic pollution and global warming generally, which causes
clouds of carcinogenic fog from the burning of fossil fuels, the worst of
which at the moment is in China,
but was a huge problem in California,
until the formation of their Air Resources Board (CARB),
when things started to improve.
THE
GUARDIAN 10 APRIL 2015 - NEW STUDY HIGHLIGHTS OCEAN SENSITIVITY
Changes to the climate have had major impacts on the oceans and the biological systems that live there. A new study sheds more light on how fast these systems respond to changes. What the authors find is that short term climate changes can require 1,000 years for recovery. This means the current harm caused to the deep oceans by the changing climate will last for many centuries to come.
The new study, published in the Proceedings of the National Academy of Sciences by Dr. Sarah Moffitt and her colleagues is novel for a number of reasons. The researchers took core samples from ocean floor regions off the coast of California. The location was chosen in part because of the exceptional synchrony between sediment archives from offshore California and ice core records from the Greenland Ice sheet.
The authors’ method was novel because they sampled many different types of creatures, not merely the single-celled organisms that are most commonly studied. In fact, the authors included Mollusca, Echinodermata, Arthropods, and Annelida samples (approximately 5,000 fossils). There was major “turnover” in these animals with only small changes to oxygen levels.

A
satellite picture supplied by the National Oceanic and Atmospheric
Administration (NOAA)
Using the ocean sediment core, the authors were able to travel back in time to the last deglaciation. They connected cooling and warming events to increases and decreases in the oxygen contained within the waters. Past events of abrupt warming, which occurred in decades to centuries and were accompanied by subsurface oxygen loss, significantly impacted the types and numbers of animals found within the sediments. Recovery from this abrupt, climate-forced disturbance can take 1,000 years.
Among the changes documented are expansions and intensification of oxygen poor regions. These regions, called “Oxygen Minimum Zones” get larger when the oceans warm. As these oxygen poor zones get larger, there is a predominance of animals that thrive in low-oxygen environments. Animals that need higher levels of oxygen suffer and die off.
It isn’t just that one ecosystem replaces another. Rather, a rich diversity of oxygen-loving biology is replaced by a much smaller diversity of low-population low-oxygen biology, and in particular single-celled organisms. In short, the ocean floor changes from a rainforest to a desert.

John Abraham
asked Dr. Moffitt about her research and she is quoted as saying: "Past events of climate warming are informative laboratories to understand the ecological consequences of abrupt changes in ocean circulation and temperature. We demonstrated that marine ecosystems can be disrupted by climate events on the timescales of multiple decades, but a subsequent recovery can take a thousand years to be complete. Fundamentally, what you can take away from this research is the vulnerability of the deep sea – truly an enormous biome on the planet – to abrupt warming."
This research is important for us today because we are causing rapid changes to the environment of the ocean and the ocean floor. We can expect these changes to impact biodiversity of the open ocean and seafloor. We now know that it will take a very long time for those biological systems to recover, basically the changes will be permanent from our standpoint. All of this makes more clear the need to take action to preserve the quality of the ocean habitat for animals and plants that live there.

WHAT
IS OCEAN ACIDIFICATION ?
Ocean acidification is the ongoing decrease in the pH of the Earth's oceans, caused by the uptake of carbon dioxide
(CO2) from the atmosphere. An estimated 30–40% of the carbon dioxide released by humans into the atmosphere dissolves into oceans, rivers and lakes. To achieve chemical equilibrium, some of it reacts with the water to form carbonic acid. Some of these extra carbonic acid molecules react with a water molecule to give a bicarbonate ion and a hydronium ion, thus increasing ocean "acidity" (H+ ion concentration). Between 1751 and 1994 surface ocean pH is estimated to have decreased from approximately 8.25 to 8.14, representing an increase of almost 30% in H+ ion concentration in the world's oceans.
Earth System Models project that within the last decade ocean acidity exceeded historical analogs and in combination with other ocean biogeochemical changes could undermine the functioning of marine ecosystems and disrupt the provision of many goods and services associated with the ocean.
Increasing acidity is thought to have a range of possibly harmful consequences, such as depressing metabolic rates and immune responses in some organisms, and causing coral bleaching. This also causes decreasing
oxygen levels as it kills off algae.
Other chemical reactions are triggered which result in a net decrease in the amount of carbonate ions available. This makes it more difficult for marine calcifying organisms, such as coral and some plankton, to form biogenic calcium carbonate, and such structures become vulnerable to
dissolution. Ongoing acidification of the oceans threatens food chains connected with the oceans. As members of the InterAcademy Panel, 105 science academies have issued a statement on ocean acidification recommending that by 2050, global CO2 emissions be reduced by at least 50% compared to the 1990 level.
Ocean acidification has been called the "evil twin of global warming" and "the other CO2 problem".
Ocean acidification has occurred previously in Earth's history. The most notable example is the Paleocene-Eocene Thermal Maximum (PETM), which occurred approximately 56 million years ago. For reasons that are currently uncertain, massive amounts of carbon entered the ocean and atmosphere, and led to the dissolution of carbonate sediments in all ocean basins.
MARINE
TECHNOLOGY NEWS, FEB 28 2015 - SATELLITES REVEAL OCEAN ACIDIFICATION
Ocean acidification can now be seen from space, highlighting an ongoing danger of
climate change and revealing the regions most at risk.
Pioneering techniques that use satellites to monitor ocean acidification are set to revolutionize the way that marine biologists and climate scientists study the ocean.
This new approach offers remote monitoring of large swathes of inaccessible ocean from
satellites that orbit the Earth some 700 km above our heads.
Seawater absorbs about a quarter of the carbon dioxide, a greenhouse gas that humans release into the atmosphere each year, mostly from the burning of
fossil fuels, according to the National Oceanic and Atmospheric Administration (NOAA).
This process has slowed the warming of the globe, as all of that carbon is locked up in the ocean's "carbon sink" rather than floating freely in the atmosphere. But when seawater takes up carbon dioxide, it becomes more acidic. According to NOAA, the surface pH of the ocean has become 30 percent more acidic since the end of the Industrial Revolution.
Rising CO2 emissions, and the increasing acidity of seawater over the next century, has the potential to devastate some marine ecosystems. Careful monitoring of changes in ocean acidity is crucial.
That acidity is not necessarily evenly distributed, however, nor is it simple to measure. Most studies rely on physical measurements taken out in the open ocean from research vessels and buoys deployed from such vessels. These measurements are spotty and expensive to collect.
Researchers at the Plymouth Marine Laboratory, the University of Exeter, Institut Francais Recherche Pour L´Exploitation de la Mer (Ifremer), the European Space Agency (ESA) and a team of international collaborators are developing new methods that allow them to monitor the acidity of the oceans from space.
"We are pioneering these techniques so that we can monitor large areas of the Earth's oceans, allowing us to quickly and easily identify those areas most at risk from the increasing acidification," study leader Jamie Shutler, a senior lecturer in ocean science at the University of Exeter, said in a statement.

Roger Harrabin (born 28 March 1955) is the
BBC’s Environment Analyst, and one of their senior journalists on the environment and energy. He has broadcast on environmental issues since the 1980s and has won many awards in print, TV and radio. He has travelled widely reporting on environment and energy and interviewed many leading figures including Margaret Thatcher, Tony Blair, Al Gore, John Kerry, Ban Ki-Moon, James Lovelock, Sonia Gandhi, Gordon Brown and Bjørn Lomborg. Aside from his speciality he covered many major general news stories including the Broadwater Farm riots, the sinking in the Thames of the pleasure boat Marchioness, the assassination of Israeli premier Yitzhak Rabin, the Soho pub bombing in 1999 and the 2003 Istanbul bombings. He is a Visiting Fellow at Green Templeton College, Oxford and an Associate Press Fellow at Wolfson College,
Cambridge.
Harrabin's reporting is dominated by risk issues. He states that often major risk issues fail to fit news criteria of novelty, drama, conflict, personality and pictures. This leads the media, he believes, to have given the wrong level of prominence to a range of risks including MMR, dirty bombs, child abduction, transport safety, exotic diseases, UK National Health Service “crisis”, the Brent Spar oil platform,
nuclear power and genetic modification. He argues that the media should find new ways of exploring long-term risk issues like preventive health and security of water, food, energy and climate.
BBC
NEWS OCT 2014 - UK CHIEF SCIENTIST WARNS OF ACID OCEANS by Roger
Harabin
The UK's chief scientist says the oceans face a serious and growing risk from man-made carbon emissions.
The oceans absorb about a third of the CO2 that’s being produced by industrial society, and this is changing the chemistry of seawater.
Sir Mark Walport warns that the acidity of the oceans has increased by about 25% since the industrial revolution, mainly thanks to manmade emissions.
CO2 reacts with the sea water to form carbonic acid.
He told BBC News: “If we carry on emitting CO2 at the same rate, ocean acidification will create substantial risks to complex marine food webs and ecosystems.”
He said the current rate of acidification is believed to be unprecedented within the last 65 million years – and may threaten fisheries in future.
The consequences of acidification are likely to be made worse by the warming of the ocean expected with climate change, a process which is also driven by CO2.
Sir Mark’s comments come as recent British research suggests the effects of acidification may be even more pervasive than previously estimated.
Until now studies have identified species with calcium-based shells as most in danger from changing chemistry.
But researchers in Exeter have found that other creatures will also be affected because as acidity increases it creates conditions for animals to take up more coastal pollutants like copper.

Shock result
The angler’s favourite bait – the humble lugworm – suffers DNA damage as a result of the extra copper. The pollutant harms their sperm, and their offspring don’t develop properly.
“It’s a bit of a shock, frankly,” said biologist Ceri Lewis from Exeter University, one of the report’s authors. “It means the effects of ocean acidification may be even more serious than we previously thought. We need to look with new eyes at things which we thought were not vulnerable.”
The lugworm study was published in Environmental Science and Technology. Another study from Dr Lewis not yet peer-reviewed suggests that sea urchins are also harmed by uptake of copper. This adds to the damage they will suffer from increasing acidity as it takes them more and more energy to calcify their shells and spines.
This is significant because sea urchins, which can live up to 100 years, are a keystone species - grazing algae off rocks that would otherwise be covered in green slime.
Dr Lewis found that at the pH expected by the end of the century, sea urchins will face damage from copper to 10% of their DNA.
Urchins are in an unfortunate group of creatures that look most likely to suffer from changing ocean chemistry.
At the bottom end of the marine animal chain, tiny creatures like plankton and coccolithophores reproduce so fast that their future offspring are likely to evolve to cope with lower pH.
At the other end of the scale are fish and crustaceans which are able to control their internal chemistry (even though some fish are affected in unexpected ways by acidification).
But the long-lived urchins are too simple to control their own body chemistry and will find it harder to adapt. They’re likely to be in trouble, along with molluscs like mussels - which provide food for predators and also perform vital services to the eco-system.
Tough, boulder corals may survive the changes, but many of the branching and table corals which provide shelter for tropical fisheries are judged unlikely to last out the century.

Slow recovery
The recent meeting of the UN’s convention on biodiversity warned that it can take many thousands of years for marine life to recover from acidification.
Dr Lewis said that it was straightforward to forecast future chemical changes to the ocean. She said predictions of future pH had drawn few of the criticisms levelled at the much more complex models of climate change.
But she warns that the biological effects of the chemical change in the oceans are harder to predict.
In her Exeter lab she is currently subjecting ill-tempered crabs to the end-of-century challenge. She plunges her hand into a seawater tank to seize the shell of one feisty specimen that does not want to be moved. It grips the water feedpipe with a vicious-looking claw.
“We think crabs should fare better with low pH than urchins do,” she tells me.
“We don't know yet how they will respond to extra availability of copper.
“Our work means we are under-estimating effects of acidification for coastal invertebrates. We are now realizing there are many indirect impacts of ocean acidification on other processes. It could be that we are facing a lot more surprises ahead.”
Dr Lewis has set herself a mission to explain the science of ocean acidification to children. Along with other ocean experts she wrote to the government urging the education department to guarantee a place for the oceans in the school science.
“It’s unacceptable that pupils can go through their entire school science career learning nothing about the oceans which cover 70% of the planet,” she says. “Ocean acidification is a fact – children should know that.”
Follow Roger on Twitter
https://twitter.com/rharrabin

EFFECTS
OF CO2 ON THE OCEANS
Although the natural absorption of CO2 by the world's oceans helps mitigate the climatic effects of anthropogenic emissions of CO2, it is believed that the resulting decrease in pH will have negative consequences, primarily for oceanic calcifying organisms. These span the food chain from autotrophs to heterotrophs and include organisms such as coccolithophores, corals, foraminifera, echinoderms, crustaceans and molluscs. As described above, under normal conditions, calcite and aragonite are stable in surface waters since the carbonate ion is at supersaturating concentrations. However, as ocean pH falls, the concentration of carbonate ions required for saturation to occur increases, and when carbonate becomes undersaturated, structures made of calcium carbonate are vulnerable to dissolution. Therefore, even if there is no change in the rate of calcification, the rate of dissolution of calcareous material increases.
Corals, coccolithophore algae, coralline algae, foraminifera, shellfish and pteropods experience reduced calcification or enhanced dissolution when exposed to elevated CO2.
The Royal Society published a comprehensive overview of ocean acidification, and its potential consequences, in June 2005. However, some studies have found different response to ocean acidification, with coccolithophore calcification and photosynthesis both increasing under elevated atmospheric pCO2, an equal decline in primary production and calcification in response to elevated CO2 or the direction of the response varying between species. A study in 2008 examining a sediment core from the North Atlantic found that while the species composition of coccolithophorids has remained unchanged for the industrial period 1780 to 2004, the calcification of coccoliths has increased by up to 40% during the same time. A 2010 study from Stony Brook University suggested that while some areas are overharvested and other fishing grounds are being restored, because of ocean acidification it may be impossible to bring back many previous shellfish populations. While the full ecological consequences of these changes in calcification are still uncertain, it appears likely that many calcifying species will be adversely affected.
When exposed in experiments to pH reduced by 0.2 to 0.4, larvae of a temperate brittlestar, a relative of the common sea star, fewer than 0.1 percent survived more than eight days. There is also a suggestion that a decline in the coccolithophores may have secondary effects on climate, contributing to global warming by decreasing the Earth's albedo via their effects on oceanic cloud cover. All marine ecosystems on Earth will be exposed to changes in acidification and several other ocean biogeochemical changes.
The fluid in the internal compartments where corals grow their exoskeleton is also extremely important for calcification growth. When the saturation rate of aragonite in the external seawater is at ambient levels, the corals will grow their aragonite crystals rapidly in their internal compartments, hence their exoskeleton grows rapidly. If the level of aragonite in the external seawater is lower than the ambient level, the corals have to work harder to maintain the right balance in the internal compartment. When that happens, the process of growing the crystals slows down, and this slows down the rate of how much their exoskeleton is growing. Depending on how much aragonite is in the surrounding water, the corals may even stop growing because the levels of aragonite are too low to pump in to the internal compartment. They could even dissolve faster than they can make the crystals to their skeleton, depending on the aragonite levels in the surrounding water.
Ocean acidification may force some organisms to reallocate resources away from productive endpoints such as growth in order to maintain calcification.
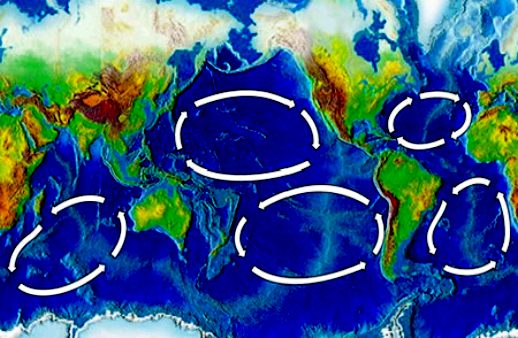
In
addition to acid oceans, the
five main ocean 'Gyres' have become giant plastic garbage patches. Does that
make you proud to be a human? Not us, we feel ashamed that this is the
legacy that we are forcing on wildlife, as we enjoy the spoils of a
runaway
unsustainable society.
OTHER
BIOLOGICAL IMPACTS
Aside from the slowing and/or reversing of calcification, organisms may suffer other adverse effects, either indirectly through negative impacts on food resources, or directly as reproductive or physiological effects. For example, the elevated oceanic levels of CO2 may produce CO2-induced acidification of body fluids, known as hypercapnia. Also, increasing ocean acidity is believed to have a range of direct consequences. For example, increasing acidity has been observed to: reduce metabolic rates in jumbo squid; depress the immune responses of blue
mussels; and make it harder for juvenile clownfish to tell apart the smells of non-predators and predators, or hear the sounds of their predators. This is possibly because ocean acidification may alter the acoustic properties of seawater, allowing sound to propagate further, and increasing ocean noise. This impacts all animals that use sound for echolocation or communication. Atlantic longfin squid eggs took longer to hatch in acidified water, and the squid's statolith was smaller and malformed in animals placed in sea water with a lower pH. However, as with calcification, as yet there is not a full understanding of these processes in marine organisms or ecosystems.
IMPACT ON HUMAN ENDEAVOR
The threat of acidification includes a decline in commercial fisheries and in the
Arctic tourism industry and economy. Commercial fisheries are threatened because acidification harms calcifying organisms which form the base of the Arctic food webs.
Pteropods and brittle stars both form the base of the Arctic food webs and are both seriously damaged from acidification. Pteropods shells dissolve with increasing acidification and the brittle stars lose muscle mass when re-growing appendages. For pteropods to create shells they require aragonite which is produced through carbonate ions and dissolved calcium. Pteropods are severely affected because increasing acidification levels have steadily deceased the amount of water supersaturated with carbonate which is needed for aragonite creation.
Arctic waters are changing so rapidly that they will become undersaturated with aragonite as early as 2016. Additionally the brittle star's eggs die within a few days when exposed to expected conditions resulting from Arctic acidification. Acidification threatens to destroy Arctic food webs from the base up. Arctic food webs are considered simple, meaning there are few steps in the food chain from small organisms to larger predators. For example pteropods are “a key prey item of a number of higher predators - larger plankton, fish, seabirds, whales" Both pteropods and sea stars serve as a substantial food source and their removal from the simple food web would pose a serious threat to the whole ecosystem. The effects on the calcifying organisms at the base of the food webs could potentially destroy fisheries. The value of fish caught from US commercial fisheries in 2007 was valued at $3.8
billion and of that 73% was derived from calcifiers and their direct predators. Other organisms are directly harmed as a result of acidification. For example decrease in the growth of marine calcifiers such as the American Lobster, Ocean Quahog, and scallops means there is less shellfish meat available for sale and consumption. Red king crab fisheries are also at a serious threat because crabs are calcifiers and rely on carbonate ions for shell development. Baby red king crab when exposed to increased acidification levels experienced 100% mortality after 95 days. In 2006 Red King Cab accounted for 23% of the total guideline harvest levels and a serious decline in red crab population would threaten the crab harvesting industry. Several ocean goods and services are likely to be undermined by future ocean acidification potentially affecting the livelihoods of some 400 to 800 million people depending upon the emission scenario.
IMPACT ON INDIGENOUS PEOPLES
Acidification could damage the Arctic tourism economy and affect the way of life of indigenous peoples. A major pillar of Arctic tourism is the sport fishing and hunting industry. The sport fishing industry is threatened by collapsing food webs which provide food for the prized fish. A decline in tourism lowers revenue input in the area, and threatens the economies that are increasingly dependent on
tourism.
Acidification is not merely a threat but has significantly declined whole fish populations. For example, In Scandinavia studies conducted on acidic water revealed that 15% of species populations had disappeared and that many more populations were limited in numbers or
declining. The rapid decrease or disappearance of marine life could also affect the diet of Indigenous peoples.
ACIDIFICATION
- ADRIATIC
- ARCTIC
- ATLANTIC - BALTIC
- BAY BENGAL - BERING
- CARIBBEAN - CORAL - EAST
CHINA
ENGLISH CH
-
GOC - GUANABARA
- GULF
GUINEA - GULF
MEXICO
- INDIAN
-
IRC - MEDITERRANEAN -
NORTH SEA - PACIFIC
- PERSIAN GULF - SEA
JAPAN
STH
CHINA - PLASTIC
- PLANKTON - PLASTIC
OCEANS - SEA
LEVEL RISE - UNCLOS
- UNEP
WOC
- WWF
AMAZON
- BURIGANGA - CITARUM - CONGO - CUYAHOGA
-
GANGES - IRTYSH
- JORDAN - LENA -
MANTANZA-RIACHUELO
MARILAO
- MEKONG - MISSISSIPPI - NIGER - NILE - PARANA - PASIG - SARNO - THAMES
- YANGTZE - YAMUNA - YELLOW
LINKS
& REFERENCE
Wikipedia
Ocean_acidification National
Geographic critical-issues-ocean-acidification BBC
News
science environment The
Guardian environment
university-offering-free-online-course-to-demolish-climate-denial http://theterramarproject.org/thedailycatch/new-study-reveals-oceans-sensitivity-to-climate-change/ http://www.bbc.co.uk/news/ http://en.wikipedia.org/wiki/Roger_Harrabin http://ocean.nationalgeographic.com/ocean/critical-issues-ocean-acidification/ http://www.bbc.co.uk/news/science-environment-29746880 http://www.marinetechnologynews.com/news/satellite-images-reveal-ocean-509822
http://en.wikipedia.org/wiki/Ocean_acidification Wikipedia
Marine_debris
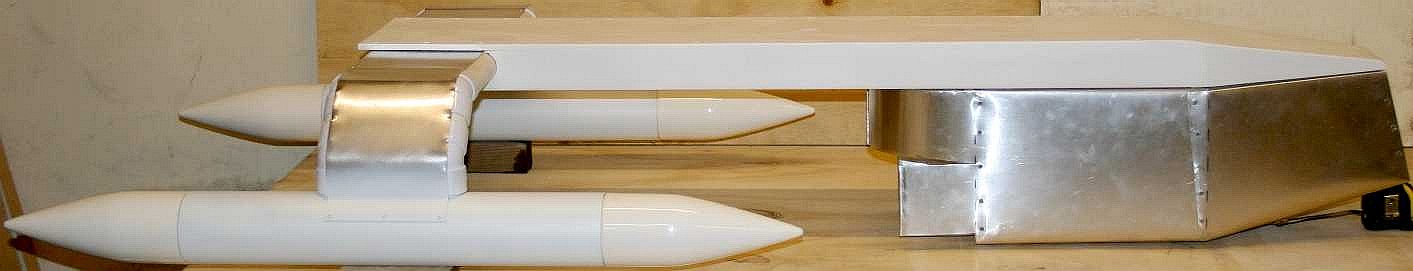
ENTERPRISE
1 - Looking somewhat like an inverted Starship,
a non-polluting vessel such as the SeaVax feasibility study above could be an ideal base engine when it comes to filtering garbage from the world's ocean
gyres. SeaVax can also monitor the ocean for acidity and other important
parameters at the same time as it trolls for plastic - and, a dual purpose
version could also be useful during major oil
spillages.
The SeaVax is a robotic
ocean workhorse that is based on a stable trimaran hull form. The
"Zero Carbon Cruiser" (ZCC) design uses no diesel fuel to cruise the oceans autonomously (COLREGS
compliant) 24/7 and 365 days a year as required. With such awesome power generating
capability, a SeaVax ZCC can be adapted to extract plastic waste from ocean garbage
patches. Several of these cleaners operating as Atlantic, Indian and Pacific
ocean fleets could make such
conservation measures cost effective, and even potentially attractive to
governments around the world - for the health of the world. Recovered
plastic could be processed to produce oil, energy or recycled products.
Better than letting fish and seabirds eat the waste and kill themselves, and
who knows how that may affect us, where seafood is an essential resource for
mankind and we are at the end of the food chain.
|








